FDA Proposes Rule on Unique Device Identification (UDI) for Medical Devices


When setting up a new Warehouse Management System (WMS), you want rack labels printed correctly and ready for easy, accurate installation. Get the most out of your WMS investment with durable, customizable warehouse labels and signs. Need help? Get in Touch ›

Durable asset tags connect your equipment with your CMMS or FM software package. Set up your program correctly the first time with properly designed tags that last in your conditions and integrate properly with your software application(s). Need help? Get in Touch ›

Uniquely and permanently identify equipment your teams need to inspect or maintain in the field to automate inspections and virtually eliminate the chance inspections are done on the wrong asset. Need help? Get in Touch ›

Explore asset tags designed for tracking work-in-process items during the manufacturing process. Need help? Get in Touch ›

Many government, military and original equipment manufacturers (OEMs) require their suppliers to mark in accordance with a specification or standard. Need help? Get in Touch ›

See why asset managers trust Metalphoto photosensitive anodized aluminum in applications where permanent identification is critical. Need help? Get in Touch ›

Explore tamper-evident and anti-counterfeit asset tracking barcode labels. Need help? Get in Touch ›

Durable, QR code labels that enable product tracking and easy product registration for consumers. Need help? Get in Touch ›

Track property, manage preventive maintenance, reduce operational costs, and more with UID labels that meet an array of government, industrial and military specifications including MIL-STD 130. Need help? Get in Touch ›

Serialized asset tags that have the durability to last the life of your education items. Our barcode labels integrate seamlessly into the leading education asset tracking software solutions and virtually eliminate errors caused by manual data collection, ensuring accurate information. Need help? Get in Touch ›

From meter tags used for meter maintenance systems to pole tags used to track inspection and treatment, utilities rely on Camcode’s highly durable anodized aluminum bar code labels. Need help? Get in Touch ›

Camcode asset tags are designed for permanent attachment a wide variety of government fixed-asset inventory items such as office equipment to outdoor street signs. Need help? Get in Touch ›

With durable medical asset tracking labels, you’ll reduce replacement costs, integrate seamlessly with asset management solutions, and eliminate manual data entry errors. Need help? Get in Touch ›

Construction, farming and mining equipment can see a lot of abuse. When tracking heavy equipment and its components, select an asset tag that is durable enough to last (and stay affixed) for the life of the equipment/component. Need help? Get in Touch ›

Manufacturers discovered long ago that Camcode asset tags and nameplates offer extremely durable asset identification and can be delivered quickly and cost-effectively. Need help? Get in Touch ›

Camcode’s broad experience in the identification products market and with ship marking are unique in the industry. Camcode produces millions of custom identification products every year and has traveled to over 250 sites worldwide to assess and mark equipment items. Need help? Get in Touch ›

Camcode barcode pole tags virtually eliminate errors caused by manual data collection, ensuring accurate information. This improves the productivity and effectiveness of a telecommunications company by reducing entry errors in the field. The results are increased revenue, lower expense and better management of risk and NESC requirements. Need help? Get in Touch ›

You can streamline picking & stocking processes and remove the guesswork of identifying the proper storage locations for inventory. Need help? Get in Touch ›

Standard asset labels don’t surive extended outdoor exposure. For assets exposed to outdoor conditions, Camcode recommends Metalphoto® photosensitive anodized aluminum. Need help? Get in Touch ›

Anodized aluminum face stock labels that are trated with our proprietary XHT process to withstand exposure to temperatures up to 1200°F. Need help? Get in Touch ›

Whether it’s hydraulic fluid, jet fuel, gasoline or a wide variety of industrial solvents, cleaners and acids, Camcode has a variety of asset tracking label materials that will remain scannable after prolonged chemical exposure. Need help? Get in Touch ›

Asset tags used in ocean environments must be resistant to corrosion from salt spray. Camcode has worked with offshore oil rigs, shipping containers, Naval vessels, and ports around the world to tag and track assets deployed on or near the ocean. Need help? Get in Touch ›

Asset tags in harsh industrial, desert or high-traffic public environments can be exposed to abrasive conditions that will render most standard asset tags unreadable. Select an asset tracking label that is proven to survive abrasive conditions. Need help? Get in Touch ›

The most durable printed aluminum substrate available, ideal for prolonged exposure to the harshest outdoor environments. The durability for which Metalphoto is known is the result of a unique manufacturing process in which a silver halide image is embedded within the sapphire-hard, anodic layer of the aluminum. Need help? Get in Touch ›

Label blanks made of the most durable CO2 laser markable aluminum substrate on the market, ready to mark onsite and available with several attachment options. Need help? Get in Touch ›

Label blanks made of the only CO2 laser-markable aluminum that produces black graphics on a natural background. Available with several attachment options. Need help? Get in Touch ›

Camcode’s Metalphoto with Teflon is perfect for applications that require resistance to paint (including CARC) or contact with strong acids or caustics. Need help? Get in Touch ›

Anodized aluminum face stock labels that are trated with our proprietary XHT process to withstand exposure to temperatures up to 1200°F. The photographic-quality bar code and graphic images are sealed within the anodic layer of the aluminum, creating a very durable, high-quality and temperature-resistant metal asset tag. Need help? Get in Touch ›

Designed specifically for applications requiring resistance to frequent cleaning with strong caustics, such as food processing, medical, laboratory, chemical, textile, petroleum and marine environments. Need help? Get in Touch ›

A popular choice for industrial and decorative applications, a robust and malleable metal that performs well in indoor and outdoor environments, offering excellent resistance to saltwater, corrosion, tarnish, chemicals and solvents, as well as extreme temperatures. Need help? Get in Touch ›

These poly-acrylic labels are strong yet flexible in many conditions, and feature UV resistance with a tamper-proof design. Need help? Get in Touch ›

Durable gloss white polyester labels with permanent pressure sensitive adhesive to clearly mark and identify indoor assets, such as office equipment. Need help? Get in Touch ›

Aneconomical plastc label option with superior pliability, performing well for interior labeling applications. Need help? Get in Touch ›
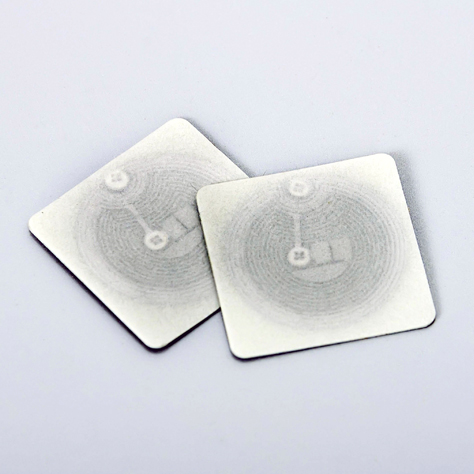
Radio Frequency Identification (RFID) tags are an ideal asset tracking system in certain applications, however before investing, consider the functionality, durability and security issues of RFID. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including MIL-STD-130 for Department of Defense UID data matrix bar code applications. Need help? Get in Touch ›

This specification covers the requirements for photosensitive anodized aluminum sheets and foils. Need help? Get in Touch ›

One of many standards that the U.S. Government has developed to guide individuals and companies within the DoD and outside the DoD on uniform engineering and technical requirements for military-unique or substantially modified commercial processes, procedures, practices, and methods. Need help? Get in Touch ›

Provides details on both the construction of the UII and the marking of items with a UII. Need help? Get in Touch ›

Provides details on the viability of using Metalphoto for marking and labeling system material components. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including Lockheed Martin UID specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including Boeing Commercial Aircraft Company specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including Honeywell, Inc. industry specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including BF Goodrich aerospace specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including SAE industry specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including NASA identification specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including Canadian Standard Association (CSA) identification specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including NATO identification specifciations. Need help? Get in Touch ›

Metalphoto satisfies wide ranging set of industrial, government and military specifications including US Department of Defense identification specifciations. Need help? Get in Touch ›

 In July 2012, the U.S. FDA announced its proposed regulatory language for a Unique Device Identification (UDI) system for most medical devices distributed in the United States. Based on legislation passed in September 2007, this proposal would establish a unique and uniform labeling system to identify U.S. medical devices throughout the supply chain, from manufacturer to provider.
In July 2012, the U.S. FDA announced its proposed regulatory language for a Unique Device Identification (UDI) system for most medical devices distributed in the United States. Based on legislation passed in September 2007, this proposal would establish a unique and uniform labeling system to identify U.S. medical devices throughout the supply chain, from manufacturer to provider.
The proposed UDI system has the potential to improve the quality of information on medical devices, including adverse events reports. This information helps the FDA identify product problems more quickly, better target recalls, and improve patient safety.
In addition to existing label requirements for medical devices, a UDI compliant label would include a device identifier, which is specific to the model of the medical device, and a production identifier, which includes the device’s recent production information, such as the lot or batch number and the serial number. This information would be displayed on the device as both human readable data and machine readable information. Typically this means the item would be encoded in linear or 2D bar codes. The information about the item will be contained in a publicly available UDI database. No patient identification information will be held in the database.
Expected benefits of Unique Device Identification include:
The FDA’s proposed rule on UDI is currently in the comment phase, with implementation projected for late 2013. Camcode will continue to research, review, and understand the information provided on the UDI initiative, and offer our thoughts and comments as new developments occur.
For more information on the FDA’s proposed rule on Unique Device Identification for medical devices, visit:
FDA.gov
OpenCongress.org
Regulations.gov
Pharmaceutical and Medical Packaging News
Our sales engineers are experts in automatic asset tracking, tagging and identification,a nd can answer all your questions. Get in touch now.
Lets Talk ›The articles here will be one category that is also tagged in this article so that users get articles similar to their interests. Blog Home ›
